Chemical energy storage lead-acid battery

Lead Acid Battery: Definition, Types, Charging Methods, And
What is a Lead Acid Battery? A lead acid battery is a rechargeable energy storage device that converts chemical energy into electrical energy. It consists of lead dioxide and

Lead-acid batteries and lead–carbon hybrid systems: A review
Therefore, lead-carbon hybrid batteries and supercapacitor systems have been developed to enhance energy-power density and cycle life. This review article provides an

Lead Acid Secondary Storage Battery
Lead Acid Battery Defined: A lead acid battery is defined as a rechargeable storage device where electrical energy is transformed into chemical energy during charging,

Lead-acid battery chemical energy storage
Are lead acid batteries suitable for solar energy storage? Solar Energy Storage Options Indeed, a recent study on economic and environmental impact suggests that lead-acid batteries are

Basics of Lead Acid Batteries
All electrochemical batteries follow the same basic principles. These are (a) we can store electrochemical energy in them, and (b) we can retrieve it later. However, more
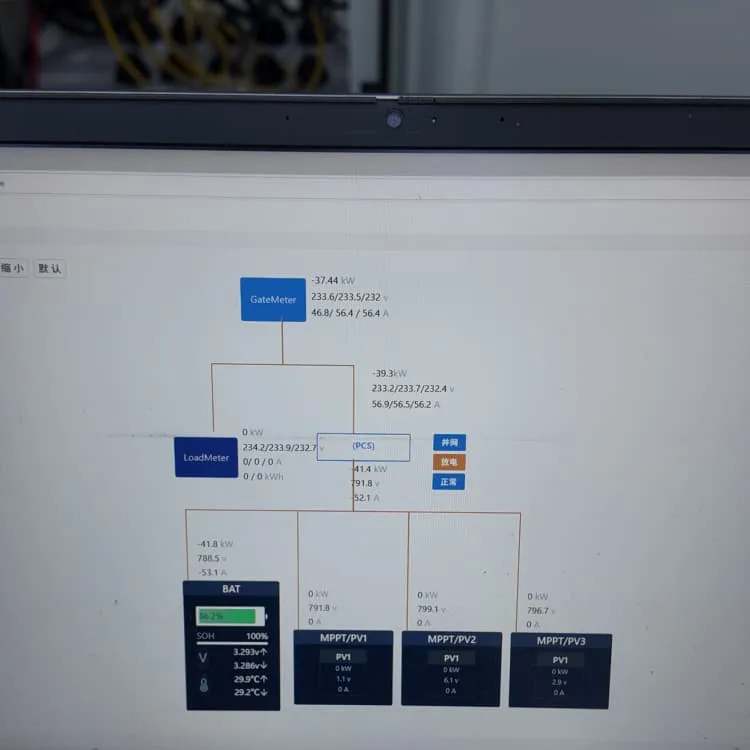
Lead batteries for utility energy storage: A review
Lead batteries are very well established both for automotive and industrial applications and have been successfully applied for utility energy storage but there are a

Microsoft Word
2.1. Lead acid battery Lead acid battery when compared to another electrochemical source has many advantages. It is low price and availability of lead, good reliability, high voltage of cell (2

Understanding the Basics: Lead-Acid Batteries Explained
At its core, a lead-acid battery embodies a sophisticated interplay of chemical reactions housed within a simple yet robust casing. Comprising lead dioxide, lead, and a sulfuric acid electrolyte

How is Chemical Energy Stored in Batteries (Proper Explanation)
Batteries store chemical energy by converting it into electrical energy. This is done by using a chemical reaction to create an electric current. The lead-acid battery is the most

Lead Acid Battery: Definition, Types, Charging
What is a Lead Acid Battery? A lead acid battery is a rechargeable energy storage device that converts chemical energy into electrical energy. It

Lead Acid Battery
Lead-acid battery charging is performed by connecting an external DC power supply to the battery for charging so that electrical energy is converted into chemical energy for storage.
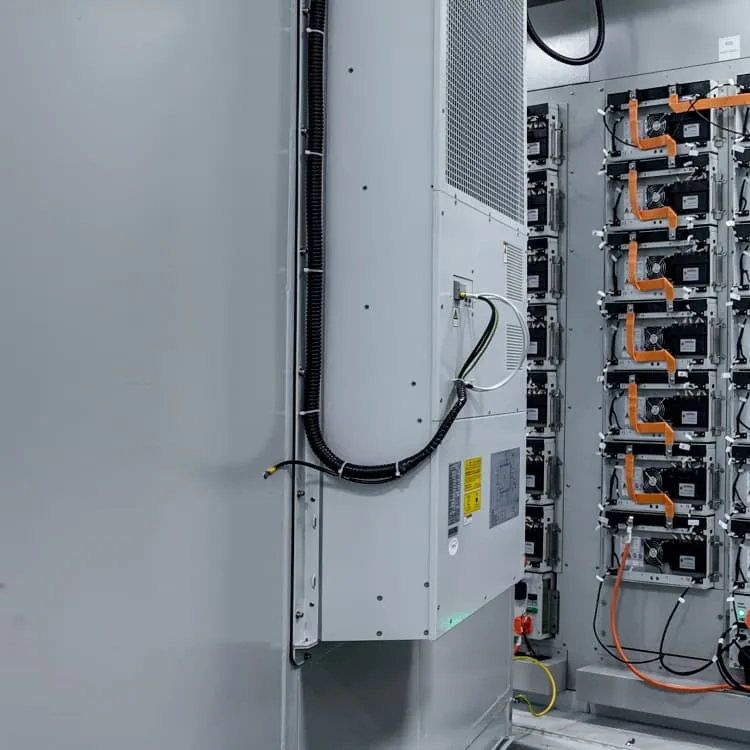
How a Lead Storage Battery is Recharged: Methods, Principles,
A lead storage battery is recharged by reversing its chemical reactions. An electrical current flows into the battery, converting lead sulfate back into lead and sulfuric acid. This

Lead-Acid Battery : Components, Reactions & Charging
Lead-acid batteries are secondary cells, meaning they can be recharged multiple times. The electrode reactions are reversible, allowing electrical energy to be stored and later discharged.

How Does Lead-Acid Battery Work?
In essence, lead-acid batteries work by harnessing the chemical reactions between lead plates and sulfuric acid to store and release electrical energy. This reliable and well

How Batteries Store and Release Energy: Explaining
Batteries are valued as devices that store chemical energy and convert it into electrical energy. Unfortunately, the standard description of

Lead-Acid Batteries: A Cornerstone of electrical energy storage
Lead-acid batteries operate on a simple yet effective electrochemical principle. They consist of two lead plates (electrodes) immersed in a sulfuric acid electrolyte solution.

Understanding the Basics: Lead-Acid Batteries Explained
At its core, a lead-acid battery embodies a sophisticated interplay of chemical reactions housed within a simple yet robust casing. Comprising lead dioxide,

Why can lead-acid batteries store energy? | NenPower
Developed in the mid-19th century, these batteries employ a chemical reaction between lead dioxide (PbO2) and sponge lead (Pb) in an electrolyte solution of sulfuric acid

What is Lead Acid Battery : Types, Working & Its
What is Lead Acid Battery? Lead acid battery comes under the classification of rechargeable and secondary batteries. In spite of the battery''s minimal

What is a Lead-Acid Battery: Everything you need to
What is a lead-acid battery? A lead-acid battery is a fundamental type of rechargeable battery. It is made with lead electrodes immersed in a

what happens when a lead storage battery discharges
When a lead storage battery discharges, several chemical reactions take place within the battery cell. These reactions result in the conversion of stored chemical energy into electrical energy,

Related information
- Solar Retrofit System
- Storage system
- Azerbaijan containerized energy storage cabinet customization
- Replacement cost of battery cabinet
- Huawei Costa Rica monocrystalline photovoltaic panels
- Russian lithium battery pack customization
- Yemen photovoltaic module project factory
- Indian energy storage machine prices
- Can energy storage devices operate independently
- Huawei Cuba inverter
- Ecuadorian photovoltaic energy storage cabinet manufacturer
- Container energy storage cabinet battery compartment
- Thailand Electric Energy Storage Project
- Costa Rica Off-Grid Inverter Prices
- Energy storage price per cabinet
- Slovakia energy storage power supply customization
- Advantages of Malta containerized energy storage tanks
- What are the advantages of independent energy storage power stations
- 100 kWh small outdoor power supply
- Western European mobile power storage vehicle manufacturers
- Mexico high power inverter
- How to use the photovoltaic energy storage cabinet site
- Photovoltaic energy storage cabinets are cheap in China